Your Cart is Empty
Free Shipping in the USA.
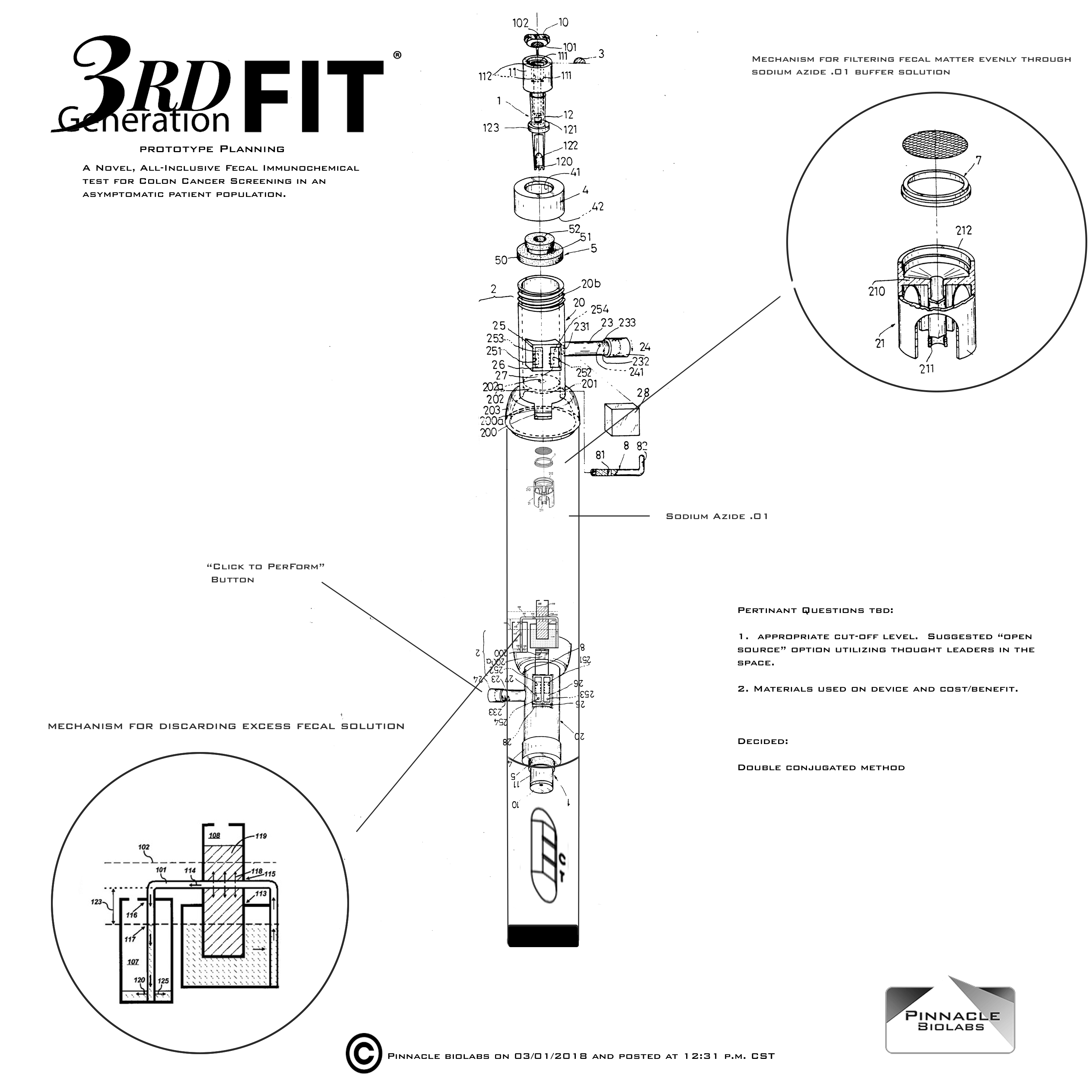
Open Source Call for Methodology and Cutoff level
Pinnacle BioLabs is in the final stages of development on a novel fecal immunochemcial test deployable to mass populations of asymptomatic persons. While the industry standard 50ng/mL cut-off levels are currently being used for most CLIA waived FIT tests, there has been significant debate over the "right" cutoff level. Recently management has decided to use an "open source" method and gain insight from thought leaders and industry experts using a roundtable approach from those who wish to glean insight.
What's at stake?
Pinnacle BioLabs Second Generation FIT® test has a large footprint as a colon cancer screening test in the United States and Europe, and has been the number one selling OTC FITtest in the world since 2014. Pinnacle BioLabs feels an inherent duty and responsibility to deliver the best possible product to physicians, laboratories, and laypersons. With help from industry experts, Pinnacle hopes to gain a consensus on the ideal cut-off level, suspension mechanism, storage, and collection method of a fecal immunochemcial test. If you have an opinion, we want to hear from you. We are in the process of establishing a roundtable event at a to-be-determinted conference to facilitate the free-thinking ideas of the world's thought leaders on the next iteration of Second Generation FIT®